Background: BMT CTN 1703 was a Phase 3 trial comparing Tac/MTX (n = 217) to post-transplant cyclophosphamide (PT-Cy)/Tac/MMF (n = 214) GVHD prophylaxis after reduced intensity conditioning unrelated donor hematopoietic cell transplant (HCT). PT-Cy significantly improved GVHD-free Relapse-Free Survival (Bolanos-Meade et al NEJM 2023). However overall survival was not significantly different and the # of Grade 2 infections was higher with PT-Cy. To investigate its biologic underpinnings 1703 was linked to a mechanistic study, BMT CTN 1801, which co-enrolled 324 pts (159 CNI/MTX 165 PT-Cy). The resulting dataset enabled an analysis of post-HCT T cell reconstitution at an unprecedented level of detail.
Methods: Samples were collected from the HCT infusion and on D+7, 14, 28, 63, 98, 180, 270, 1- and 2-yr. We extracted DNA (2,225 samples) and performed Adaptive Biotech β-strand T Cell Receptor (TCR) Immunosequencing (TCR-Seq). We profiled 44,077 (median) TCRs/sample resulting in 215,155,719 T cells analyzed. We evaluated multiple TCR diversity measures longitudinally and before/after cGVHD and infections. Each measure evaluates a specific aspect of TCR diversity, including Simpsons Clonality (focuses on the most expanded clones) Diversity Slope (mid-range clones) Richness (both mid-range and less-expanded clones) and Singletons (the TCR-Seq equivalent of naïve T cells). This analysis was robust to the full range (1000-50,000) of down-sampled TCRs sequenced.
Results: We identified major differences in TCR reconstitution in Tac/MTX vs PT-Cy that begin early and persist through 2 yr post-HCT. These resulted in a significantly less diverse TCR repertoire with PT-Cy with substantial implications for its efficacy and toxicity.
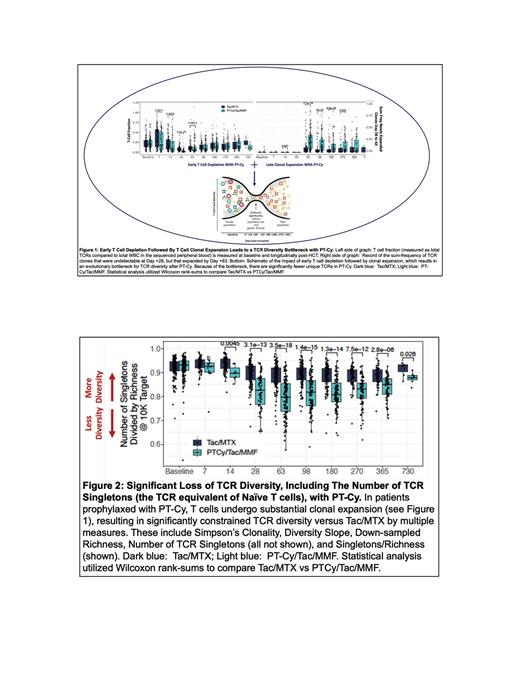
The most salient differences are: (1) PT-Cy caused significant in-vivo T cell depletion early post-HCT (% T cells/total WBC at D+28: 8.2% (Tac/MTX) vs 2.2% (PT-Cy) p=2.2x10 -23). (2) The remaining donor T cells in PT-Cy undergo substantial clonal expansion ( Fig 1) resulting in significantly constrained TCR diversity measured by Clonality, Slope, Richness, Singletons (not shown) and Singletons/Richness ( Fig 2) which began early (D+14) and persisted for 2 yr. (3) Linked T cell depletion and clonal expansion resulted in a TCR diversity bottleneck after PT-Cy ( Fig 1). Due to the bottleneck, while the fraction of T cells originating from the transplant infusion at =/> 1 yr was similar between arms (18% vs 24%, p=0.7) there were significantly fewer Singleton TCRs in PT-Cy, suggesting a reduction in naïve T cells ( Fig 2), an outcome that has critical consequences for both cGVHD and infection.
Thus while cGVHD onset with Tac/MTX was linked to a significant drop in TCR diversity due to singleton clonal expansion (median log-2 fold change in singletons with Tac/MTX = -0.25 p = 0.01) this drop was not observed with PT-Cy (log-2 fold change = -0.05, p = 0.1). Along with the significantly decreased # singletons at all time-points in PT-Cy ( Fig 2) this suggests that while cGVHD after Tac/MTX is linked to substantial naïve T cell expansion, the same is not true with PT-Cy, identifying a novel mechanism for cGVHD control with PT-Cy.
The substantially fewer singletons in PT-Cy vs Tac/MTX may also increase the risk of Grade 2+ infections. T cell responses to new infections rely on antigen recognition/effector maturation of naïve (singleton TCR) T cells. After infection, the TCR repertoire became less diverse for both Tac/MTX and PT-Cy (log-2-fold singleton change -0.17 and -1.2 p = 0.0006 and 0.01) indicating that singletons in both cohorts were capable of expanding to pathogens. However the markedly decreased # singletons in PT-Cy at all timepoints was associated with significantly fewer singletons prior to infection (mean 3979 at 5K read-depth for Tac/MTX vs 2820 for PT-Cy p<0.001). This singleton deficit would place PT-Cy patients at higher risk of missing the critical mass of TCRs required to respond effectively to infections, consistent with their increased rate of Gr 2 infections.
Conclusions: We performed longitudinal TCR-Seq on >200 million T cells from 324 HCT patients, representing the largest post-HCT TCR-Seq analysis in the history of the field, and enabling novel insights into TCR dynamics. These data identify a previously unappreciated global TCR diversity bottleneck with PT-Cy that simultaneously protects patients from cGVHD and places them at higher risk of infectious complications.
Disclosures
Kean:Merck EMD Serono: Research Funding; Novartis: Research Funding; Tessera: Research Funding; Vertex: Consultancy, Membership on an entity's Board of Directors or advisory committees; Vor: Other: Materials Transfer Agreement; Bristol Myers squibb: Patents & Royalties: royalties for clinical trial results., Research Funding; HiFiBio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Mammoth: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Regeneron: Research Funding; Gilead: Research Funding. Schmalz:Adaptive Biotechnologies: Current Employment. Bien:Adaptive Biotechnologies: Current Employment. Scheffey:Adaptive Biotechnologies: Current Employment. Sanders:Adaptive Biotechnologies: Current Employment. Robins:Adaptive Biotechnologies: Current Employment. Bar:Bristol Myers Squibb: Current Employment. Jenq:MaaT Pharma: Membership on an entity's Board of Directors or advisory committees; Da Volterra: Consultancy; Prolacta: Membership on an entity's Board of Directors or advisory committees; Kaleido: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Seres: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; LISCure: Membership on an entity's Board of Directors or advisory committees; Karius: Membership on an entity's Board of Directors or advisory committees; Microbiome DX: Membership on an entity's Board of Directors or advisory committees; Merck: Membership on an entity's Board of Directors or advisory committees. Levine:X4 Pharmaceuticals: Consultancy; Mesoblast: Consultancy; Sanofi: Consultancy; Kamada: Consultancy; Inhibrx: Consultancy; Genentech: Research Funding; Mesoblast: Research Funding; Incyte: Research Funding; Viracor: Patents & Royalties: GVHD biomarker patent.; Bluebird Bio: Consultancy; Editas: Consultancy; Equillium: Consultancy; Incyte: Consultancy. Logan:Enlivex: Consultancy. Murthy:CRISPR Therapeutics: Membership on an entity's Board of Directors or advisory committees; Senti Biosciences: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Bavarian Nordic: Membership on an entity's Board of Directors or advisory committees. Sandhu:Autolus Therapeutics: Consultancy; City of Hope Medical Center: Current Employment. Al Malki:Tscan: Consultancy. Elmariah:Bristol Myers Squibb: Research Funding. Shaffer:Hansa Biopharma: Consultancy; Gamida Cell: Consultancy, Research Funding. Rezvani:Pharmacyclics.: Research Funding. Holtan:Vitrac: Research Funding; Incyte: Research Funding; Ossium: Consultancy; Sanofi: Research Funding; CSL Behring: Other: Endpoint Adjudication Committee. Perales:Incyte: Consultancy, Honoraria, Research Funding; Kite: Consultancy, Honoraria, Research Funding; Allovir: Consultancy; VectivBio AG: Consultancy, Honoraria; Exevir: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Celgene: Honoraria; Astellas: Consultancy, Honoraria; Medigene: Consultancy, Other; Sellas Life Sciences: Consultancy; Servier: Other; Adicet: Honoraria; Vor Biopharma: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria; Equillium: Consultancy, Honoraria; Caribou: Consultancy, Honoraria; NexImmune: Consultancy, Current equity holder in publicly-traded company; Syncopation: Honoraria; DSMB: Other; Miltenyi Biotec: Honoraria; BMS: Consultancy, Honoraria; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; AbbVie: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Cidara Therapeutics: Consultancy, Other; Allogene: Research Funding. DeWolf:Atreca: Current equity holder in publicly-traded company, Other: Spouse is an equity holder.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal